After many months of research and interaction with the FDA, Molekule Air Mini, Air Mini+, Air Pro and Air Pro RX have received FDA 510(k) clearance as a Class II medical device.
Air Mini, Air Mini+ and Air Pro are already trusted to clean the air in homes and businesses around the world, and are now also intended for medical purposes to destroy bacteria and viruses in hospitals and other healthcare settings, including home healthcare as well.
To help understand the specifically cleared medical purposes for these devices, please refer to Indications for Use.
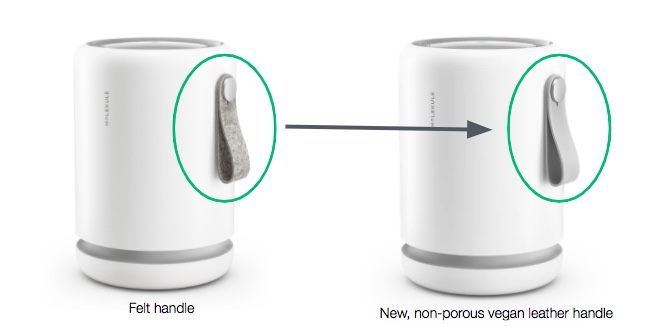
Look for enhanced FDA labeling and instructional material on healthcare use and 510(k) compliance on new purchases of Molekule Air Mini, Air Mini+ and Air Pro purifiers. The hardware and underlying PECO technology remain unchanged. Air Mini now also has a non-porous, vegan-leather handle for easier cleaning in medical settings.
Molekule's other air purifier (Molekule Air) have not been expanded for medical use, however it's important to note that PECO technology underlies all of Molekule's products.
Interested in learning more? Check out our blog posts:
Air Pro Receives Class II Medical Device Designation from the FDA
Molekule Air Mini Receives FDA Clearance to Destroy Viruses and Bacteria